Bilateral cooperation
project MEdC-JSPS
Project title:
Enhancement of depollution efficiency of the residual waters by electrical
discharges (EDERWED)
Partners:
National
Institute for Laser, plasma and Radiation Physics: Dr. C.P.Lungu
Period: April 30th
to may 30th 2009
Research
report
The working stage was done under the bilateral
agreement between JSPS,
Pulsed
discharges were produced underwater using a discharge cell manufactured at
National Institute for Laser, Plasma and Radiation Physics,
Non-thermal plasma processing in aqueous solution by applying a high voltage is considered to be an energy efficient method for the production of highly active species necessary for depollution. Different types of electrical discharges (AC, DC, pulsed) generated either directly in water or above the water surface have been studied as possible methods for water treatment. It has been demonstrated that the pulsed high voltage discharges in water generate plasma that initiate a variety of physical and chemical effects as high electric field, intense ultraviolet radiation, overpressure shock waves and, especially, formation of various reactive chemical species such as radicals (OH*, H*, O*, H2O*) and molecular species (H2O2, H2, O2). These effects have various important roles in different application regions in the liquid and the magnitude of their contributions strongly depends upon the energy of the discharge. In order to apply high voltage electrical discharges to improve environmental problems it is necessary to consider the types of chemical reactions initiated by the discharge and the effects of the physical processes on the promotion of desirable chemical reactions. The oxidation potentials of O and OH radicals are higher than that of ozone, typically produced in underwater discharges. However, the lifetimes of these radicals are so short to utilize them effectively. Therefore, the direct radical generations by plasmas are widely researched, as for example pulsed corona discharge or pulsed arc discharge in water, and discharge inside bubbles.
We previously studied aqueous solution metallic compounds abatement using high voltage pulsed discharge. In this paper, a stream of bubles is generated within the solution adjacent to the cathode. A potential difference is applied between the cathode and anode such that a glow discharge is formed in the bubble region and a plasma of ionised and excited gas molecules is formed within the gas bubles and the liquid solution.
The bubble gas reactor was a cylinder cell with two-liter capacity, and the electrodes were fixed in the top part of the reactor, made of polyimide as shown in Fig.s 1 and 2 The cathode, a perforated stainless steel cylinder of 6 mm diameter and 20 cm length, immersed into an aqueous solution by a depth of 10 cm, no insulation on the side dipped in the solution, was set in the chamber of the reactor. The anode was a similar stainless steel cylinder placed at a distance of 4 mm from the cathode. The current was supplied by a PEKURIS (+/- 4kV, 0-7A) pulsed power supply and homemade high voltage pulsed HV power supply, which provided a maximum current of 15 A at voltage up to 20 kV for the part of the experiment performed an NILPR.
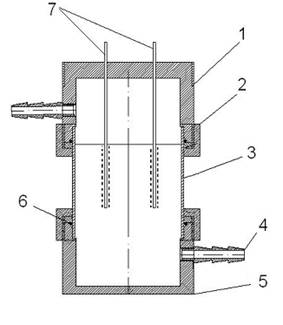
Fig.1 Schematic diagram of the gas bubble generator. (1) the top part of the reactor made of polyimide;
(2) counter piece of the part (1); (3) Pyrex tube; (4) exhaust water nozzle; (5) the bottom part of reactor; (6) rubber seal; (7) electrodes.
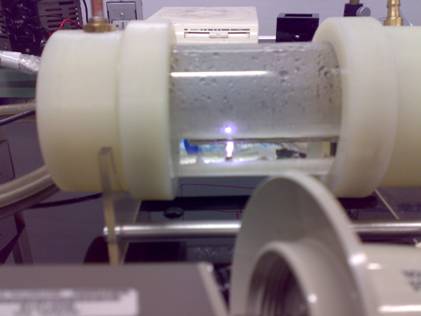
Fig.2 The photograph of the discharge cell working with Ar bubbling gas
in the experiment performed at
The used
repetitive frequency of the power
supply generator was 10-30 kHz. The current passing through the electrodes and
the voltage across the circuit were measured by a Tektronix TDS 2002
oscilloscope. The flow rate of the bubbling gas introduced through the perforated cathode was measured by a gas flow rate
meter (the used rates were between 0.1 and 2l/min). The liquid solutions used
in the experiments were distilled water and residual water with metallic
components. Pressure was atmospheric and the reaction time was 20 min. In the
beginning, the Ar and N2+O2 were introduced at different
flow rates then the applied voltage across the two electrodes was slowly
increased. In a the separate experiment performed at NILPRP, an O2
gas was used as bubbling gas, in this case the applied voltage was 25-27 kV at
a frequency of 260 Hz.

Fig.3 The full set-up of the experiment
performed at
The shape of
the voltage and current applied to the disharge cell, measured with the
Tektronix oscilloscope are show in Fig.4.
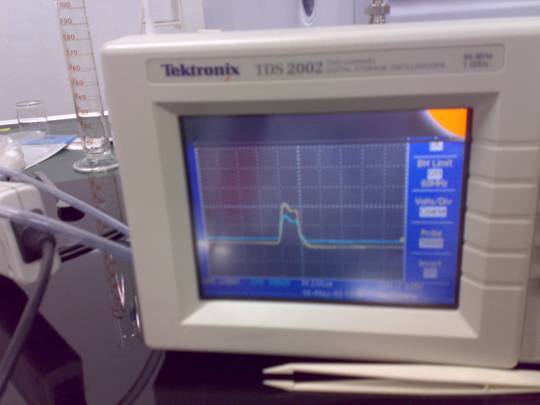
Fig. 4 The applied voltage and current pulse shapes.
A typical
optical emission spectrum acquired using an AVANTES spectrometer is presented
in Fig.5. Active species as Ar*, OH*, H*, O*, H2O* were identified.
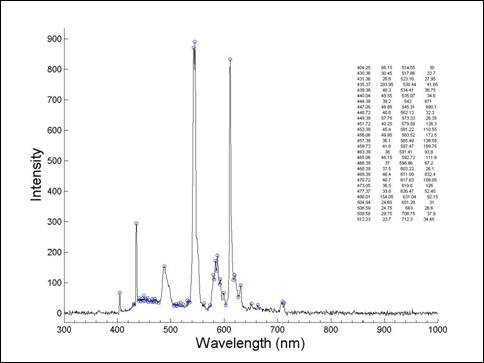
Fig. 5 Typical emission spectrum acquired
using an AVANTES optical emission spectrometer.
The detailed mechanism of
the initiation of the discharges in water is still not fully understood. Two
types of theories, i.e. electronic theories and thermal breakdown (bubble) theories, have been proposed to explain
the initiation of the discharges in water. According to the electronic theories
the free electrons are accelerated
under the applied electric field and may collide with and ionize the ambient
molecules, thus producing more free electrons (electron avalanche) and leading
to breakdown in water. According to the thermal breakdown (bubble) theories the
current in the high-field region causes heating and vaporization of the liquid,
forming bubbles. Gas breakdown occurs within each bubble, causing
further heating and growth of the bubble until complete breakdown of the gap
occurs. A single streamer has a fraction of a millimetre diameter and can
propagate to a distance of more than a centimeter in water. The electron
density in the case of streamers in water
increase with the solution conductivity and is of the order of 1018
cm−3 when the
conductivity is 210 S cm−1. The glow discharge causes the generation of the plasma in
the gas bubbles.
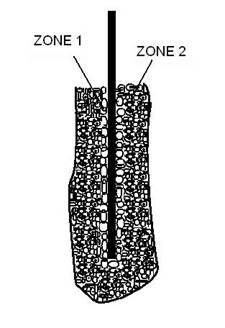
Fig. 6 Formation of a bubble sheath
around the cathode
Fig.6 shows the formation of a bubble sheath
around the cathode. As shown in figure, it was observed the formation of two
distinct zones during pulsed glow discharge. In zone 1 where the glow discharge
clusters are present, there is a plasma envelope that directly covers the
cathode surface. The plasma interacts with the cathode surface in a process
similar to ion plating and deposition of the metals contained in the liquid
solution occurs. A film is formed through nucleation and growth on the cathode
surface. Zone 2 is a plasma-chemical reaction zone, which forms the interface
between the electrolyte and zone 1. This zone envelope the plasma deposition
zone and is often clearly visible as a separate region with an unclear
appearance.
Dissociation, and possibly ionization of the
liquid solution components occur in the outer zone 2. The species could be
transferred from the outer zone 2 to inner zone 1 by the electric field
strength, diffusion and convection. Deposition on the cathode occurs for as long
as these conditions are maintained and the metallic components are available in
the electrolyte. After the glow discharge ignition the temperature of the
electrodes increases rapidly. The temperature of the electrolyte must be
maintained within acceptable limits. A method to perform the cooling is the
introduction of a pumped cooling system of the liquid solution as well as the
increase of the oxygen gas flow rate within the perforated cathode.
In the gas bubbling phase, the high-voltage
pulse was applied between the perforated
electrodes. The pulsed discharge plasma was generated in the gas phase;
simultaneously, the plasma channel was permeated into the water phase
accompanied by the gas bubbles. The water phase plasma produced a lot of active
species, UV light and high-energy electrons.
After 20 minutes of plasma treatment, the concentration of some heavy
metals diminished drastically (Pb from 0.27 mg/l to <0.10 mg/l, Sb from 0.8
mg/l to <0.1 mg/l, etc). The active species (H*, O*) were identified by
optical emission. The water composition (Table 1) before and after plasma
treatment was analyzed using mass spectrometry. (Mass spectrometer Optimass
8000 of KROHNE Messtechnik GmbH & Co. KG,
ELEMENT
|
SAMPLES |
||||
|
|
NON-TREATED |
Sample 1 |
Sample 2 |
Sample 3
|
Sample 4 |
|
Cu |
48,68 |
30,57 |
29,02 |
30,41 |
18,90 |
|
Cd |
12,71 |
4,35 |
4,57 |
3,53 |
2,79 |
|
Cr |
13,58 |
10,98 |
5,77 |
5,04 |
5,07 |
|
Mo |
4,21 |
3,14 |
3,23 |
3,04 |
2,12 |
|
Ni |
44,65 |
38,54 |
37,49 |
36,05 |
33,90 |
|
Pb |
24,67 |
3,31 |
4,97 |
2,11 |
2,00 |
|
Sn |
430,06 |
16,27 |
6.65 |
5,97 |
2,6 |
|
Zn |
21,85 |
15,37 |
9,33 |
10,0 |
2,10 |
|
Ba |
18,71 |
14,86 |
13,76 |
10,48 |
9,50 |
|
Se |
397,53 |
461,76 |
347,6 |
350,10 |
450,0 |
|
Hg |
15,15 |
6,54 |
- |
- |
- |
|
As |
5,29 |
4,46 |
4,38 |
4,41 |
3,90 |
|
Rb |
93,62 |
81,17 |
80,95 |
86,02 |
84,02 |
|
V |
2,66 |
2,29 |
2,50 |
2,39 |
2.17 |
|
Tl |
8,84 |
7,48 |
8,01 |
7,04 |
7,09 |
Table 1. Aqueous composition before and after plasma underwater discharge treatment
Metal concentration was reduced by the plasma treatement, the decomposition rate became high with increasing the gas flow rate. The decomposition energy efficiency was higher with the increases of the gas flow rate, and it was higher when the hole diameter was smaller. From these results, it seems that high-speed radical flow is the most crucial factor in metal-metal electrode system because of the very short lifetime of O* radical. Water conductivity plays an important role in the generation of underwater discharges and on the production of chemically active species. In deionized water, the discharge is relatively weak. A certain concentration of ions (conductivity in the range of 10–80 μS cm−1) enhances conduction, resulting in a stronger discharge, higher current flow, longer streamer length and an increase in the production of chemically active species. An increase in the water conductivity (further from the optimum value of 10–80 μS cm−1) results in a faster compensation of the space charge electric field on the streamer head (shorter streamer channel length) and a decrease in rate of production of chemically active species
We can conclude that underwater discharge technique cold be more
effective, cheaper and environmentally friendly than conventional water
treatment techniques. Further developments in the area of water treatment by
electrical discharges need to be performed. The application of pulsed high
voltages during water purification/ozonation may result in better dispersion of
ozone in water and faster conversion of ozone into free radicals, which may
lower the cost of the ozonation processes. The treatment of aqueous solutions
containing levigates is being tested on an industrial scale. Also, an intense
study of the dc and pulsed discharges as were demonstrated in aqueous medium
can furnish more interesting data.
On
May 14th I visited Technical University of Toyohashi, the research
group of professor Hirofumi Takikawa. I presented a one-hour lecture with the
title of “Multifunctional film preparation using thermionic vacuum arc method”.
I visited the research facilities of this group and I was impressed by the high
level of research and apparatuses used for plasma production and film
characterization.

Fig.7
Atmospheric pressure plasma torch for surface treatment (Technical University
of Toyohashi)

Fig.8
Gliding arc discharge working at atmospheric pressure. (
The research results obtained during the working stage were presented as
invited lecture to the International Balkan Workshop on Applied Physics, to be
held in